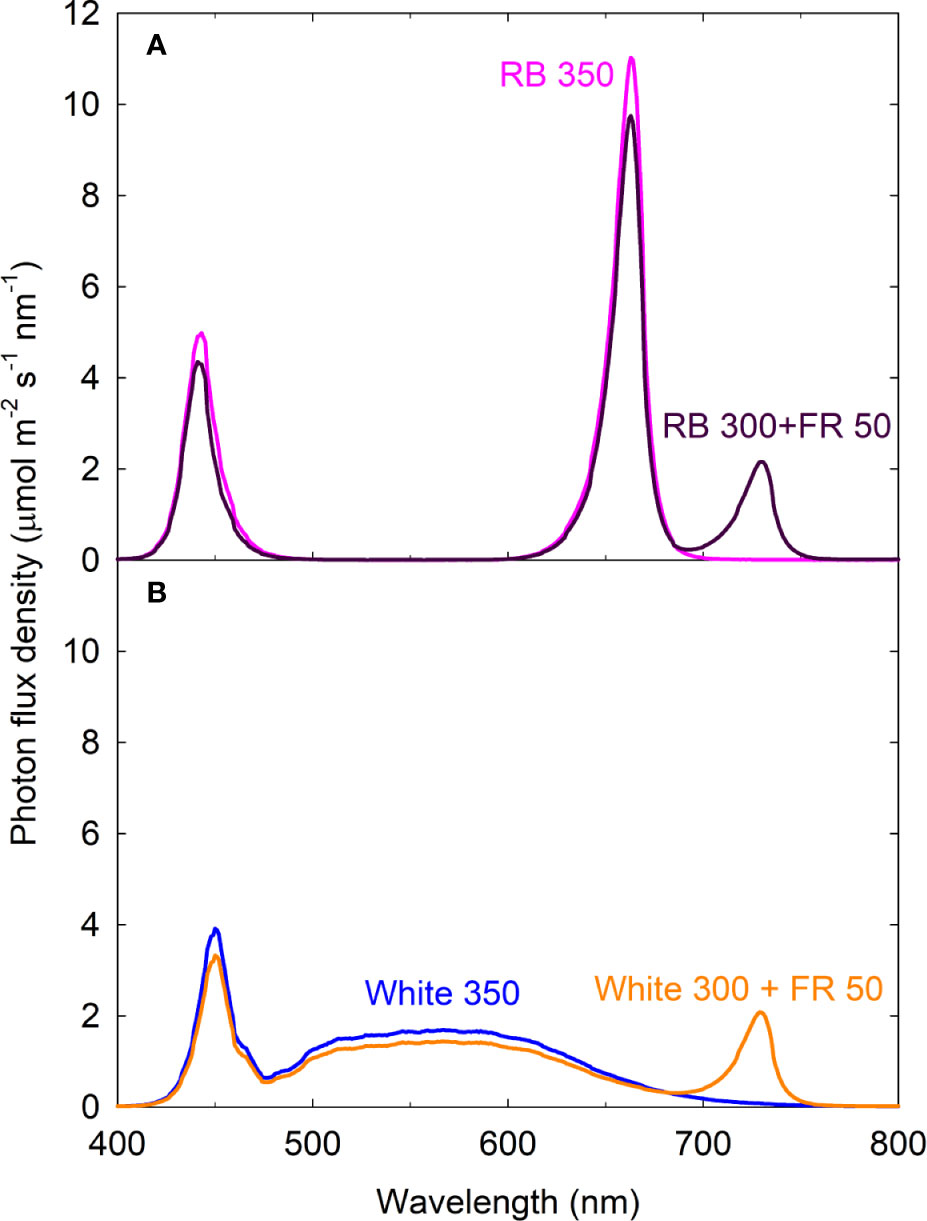
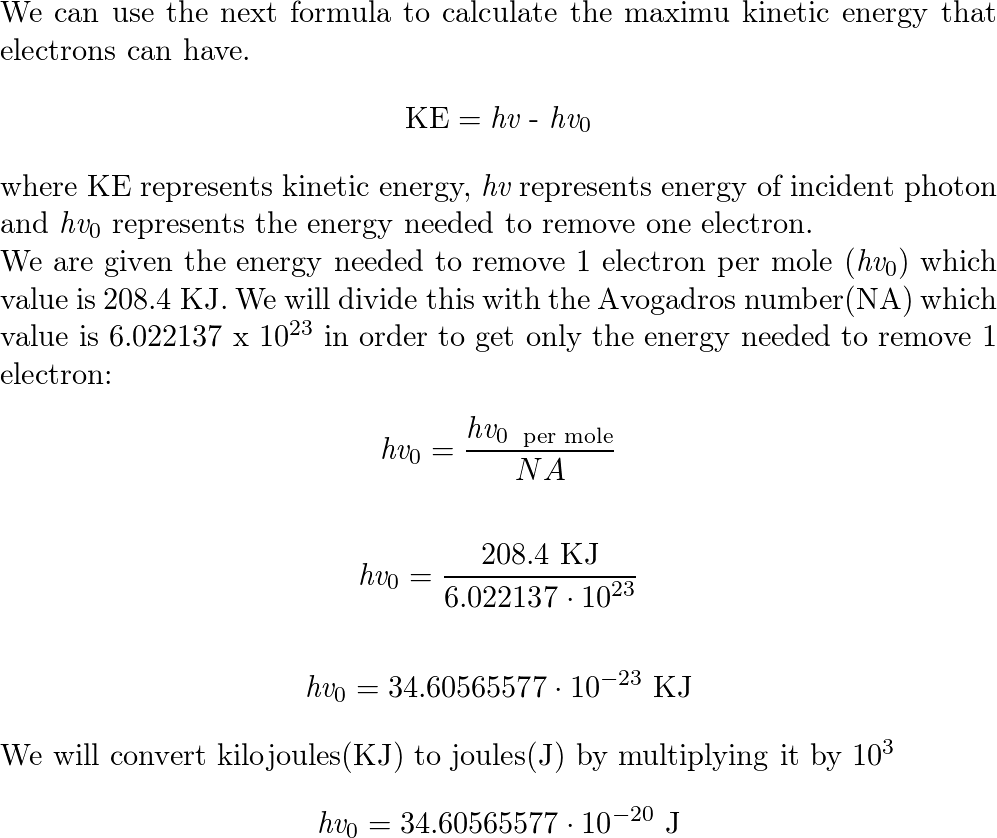
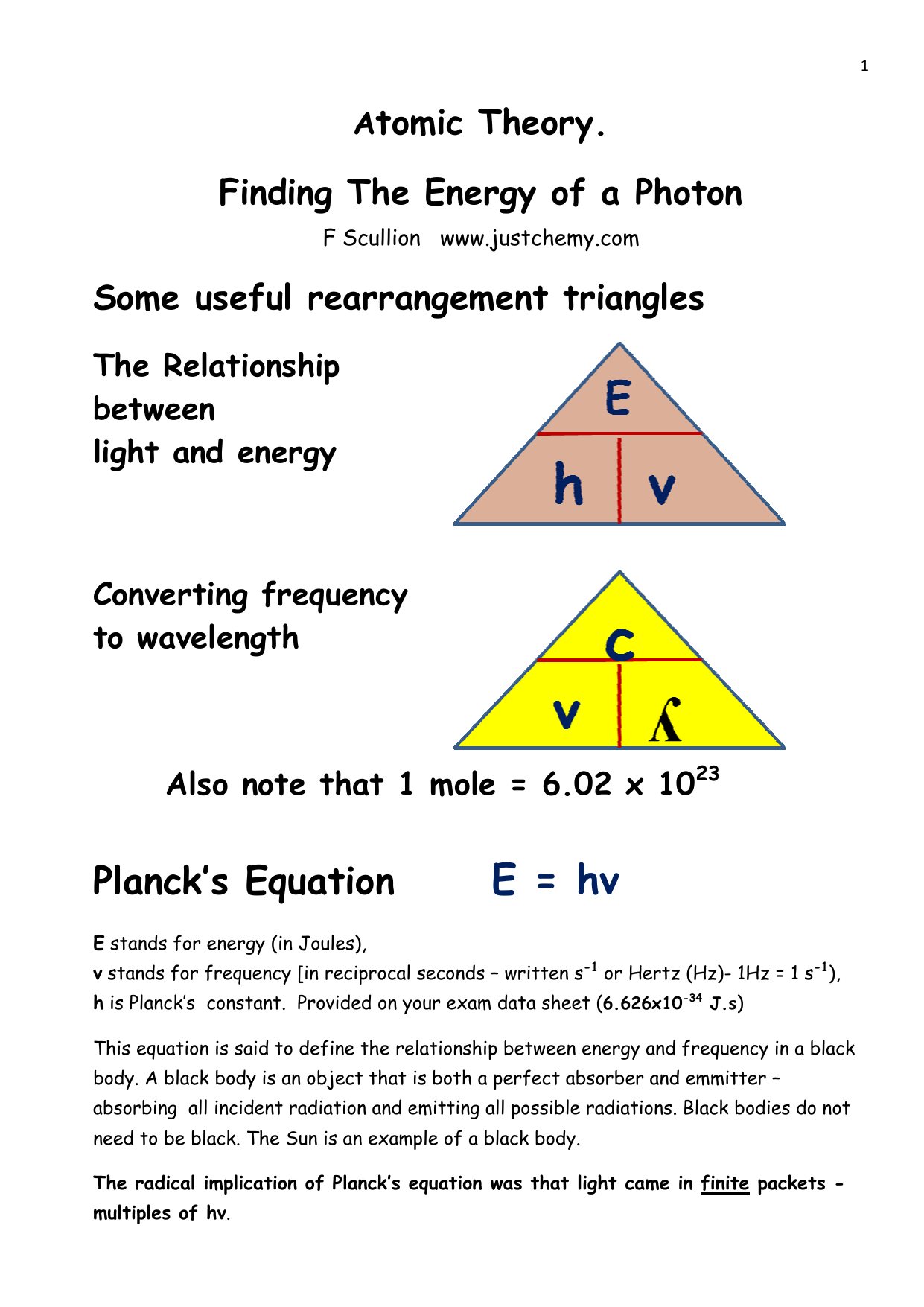
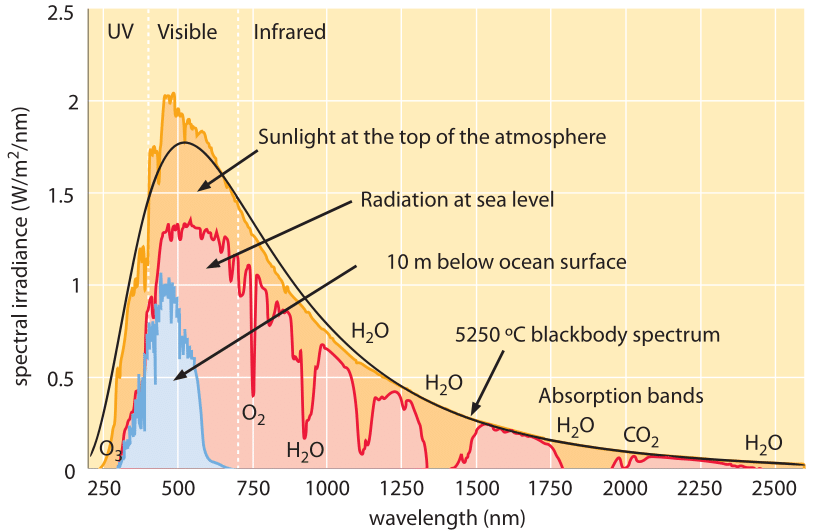
English) Calculate the energy of one mole of photons of radiations whose frequency is 5 X 10^ 14 Hz - YouTube

Calculate the energy of one mole of photons of radiation whose frequency is `5 xx 10^(14) Hz`. - YouTube

Calculate the energy in kilocalorie per mole of photons of an electromagnetic radiation having a wavelength of 7600 A.A. 33.56B. 37.56C. 47.35D. 42.35

Calculate the energy of one mole of photons of radiations having wavelength 300 nm. (c=3xx10^(8) mS^(-1), h=6.626xx10^(-34)JS).

If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 × 10^7ms^-1 , calculate the energy

calculate energy of one mole of photons of radiation whose frequency is `5xx10^(14) hz`... - YouTube

Light of wavelength 2475 A in incident on barium. Photoelectrons emitted describe a circle of radius 100cm by a magnetic field of flux density 1√(17) × 10^-5 Tesla. Work function of the