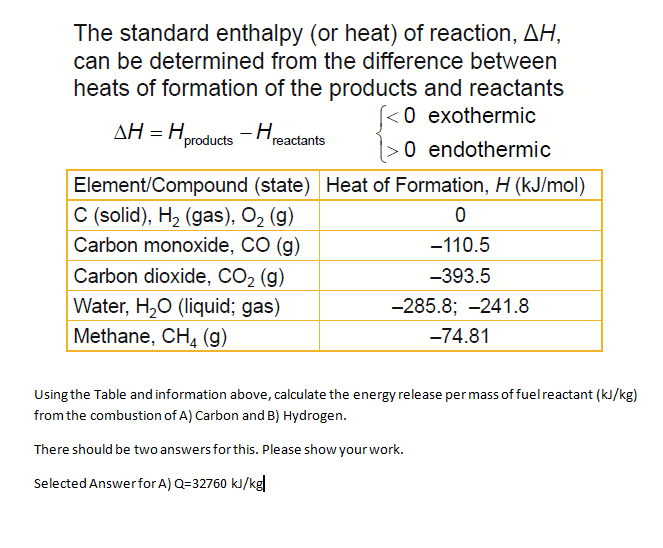
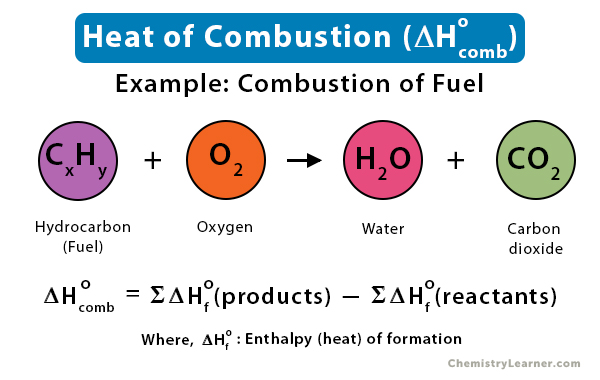
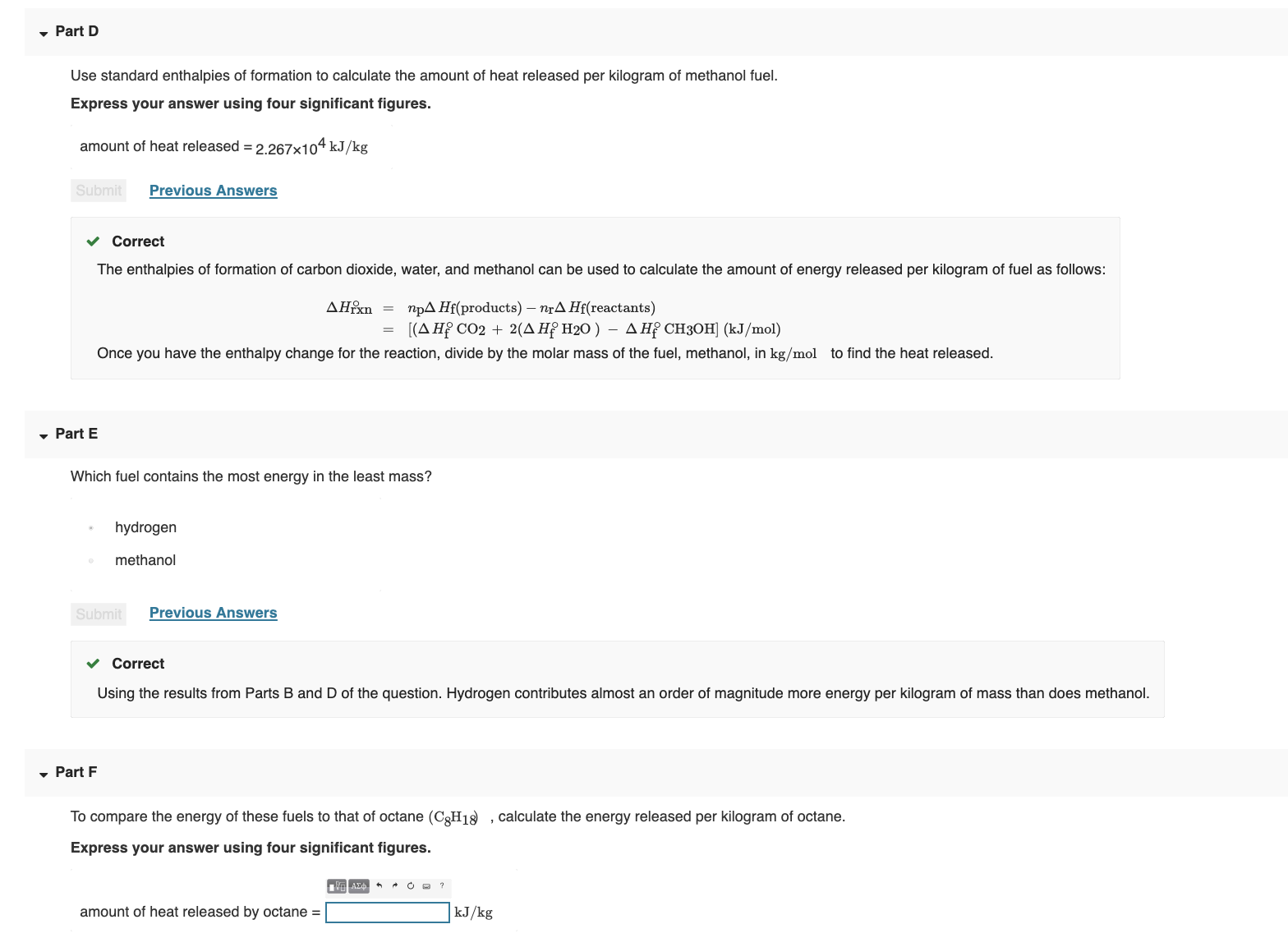
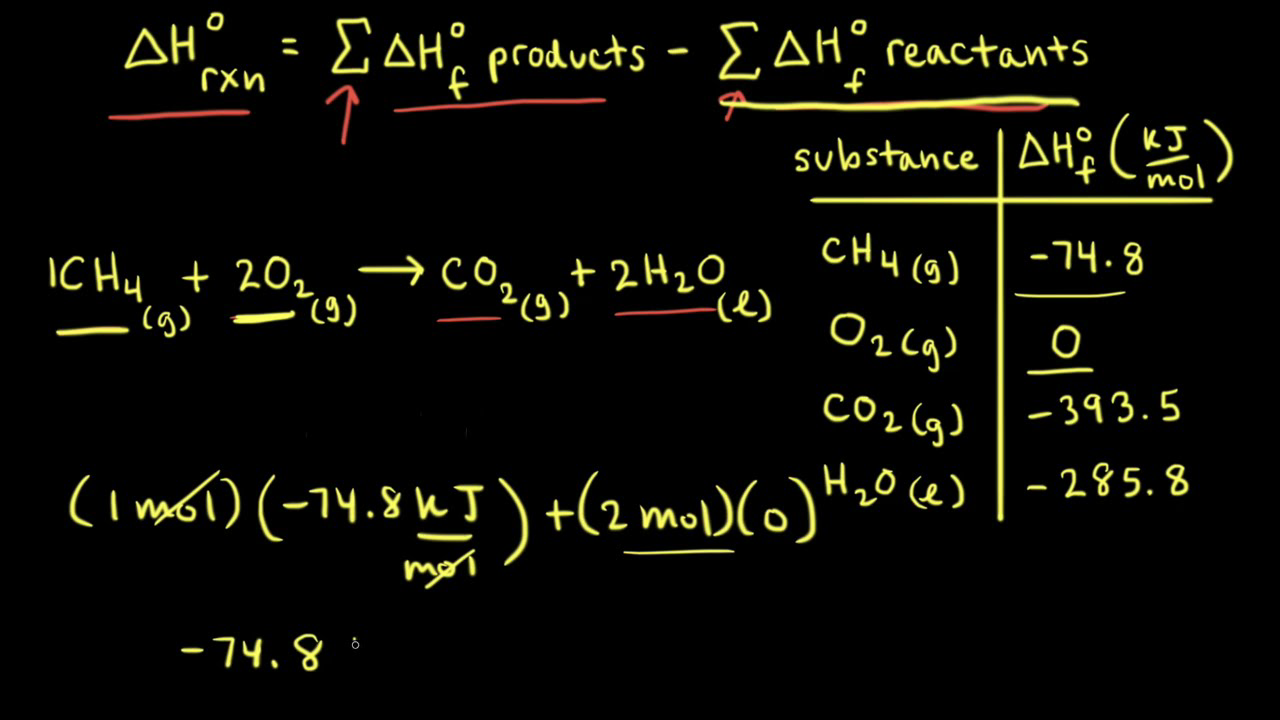
Given the following equations calculate the standard enthalpy of the reaction, 2Fe(s) + 3/2 O2(g) → Fe2O3(s) H^o = ? (i) 2Al(s) + Fe2O3(s) ⟶ 2Fe(s) + Al2O3(s) H^o = - 847.6

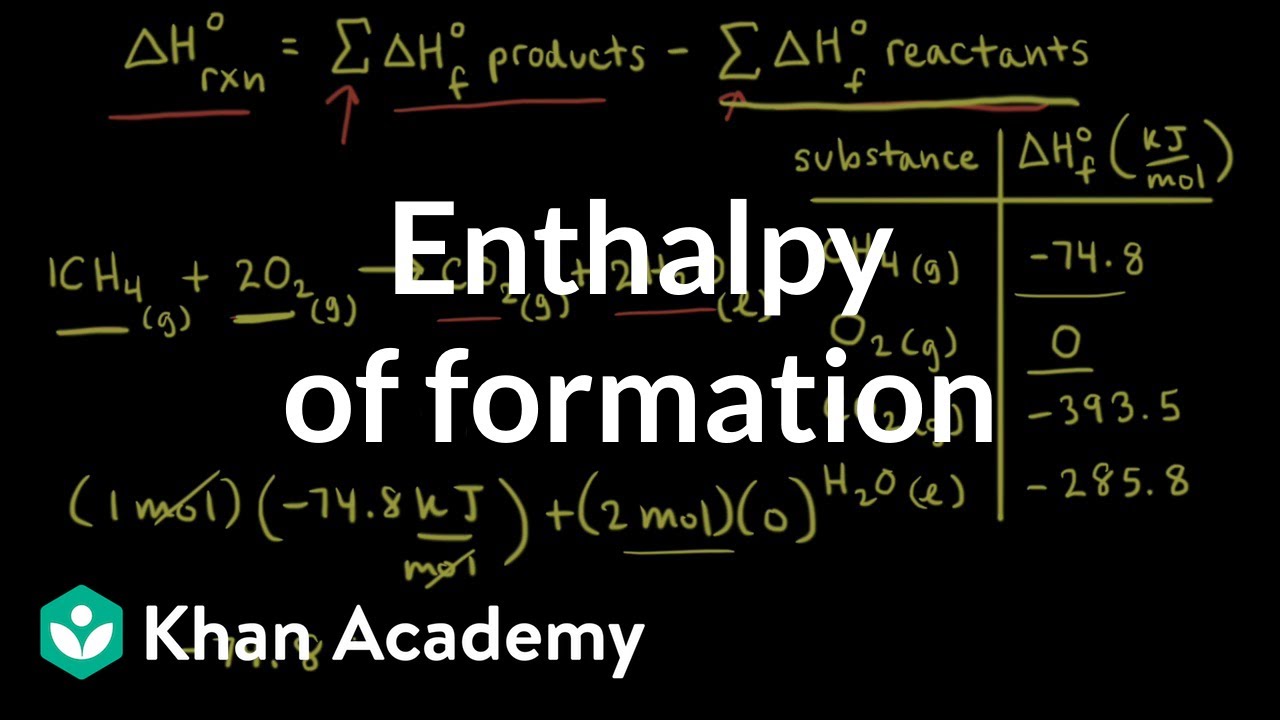
Enthalpy of combustion of carbon to CO2 is -393.5KJ mol-1. Calculate the heat released upon..... - YouTube

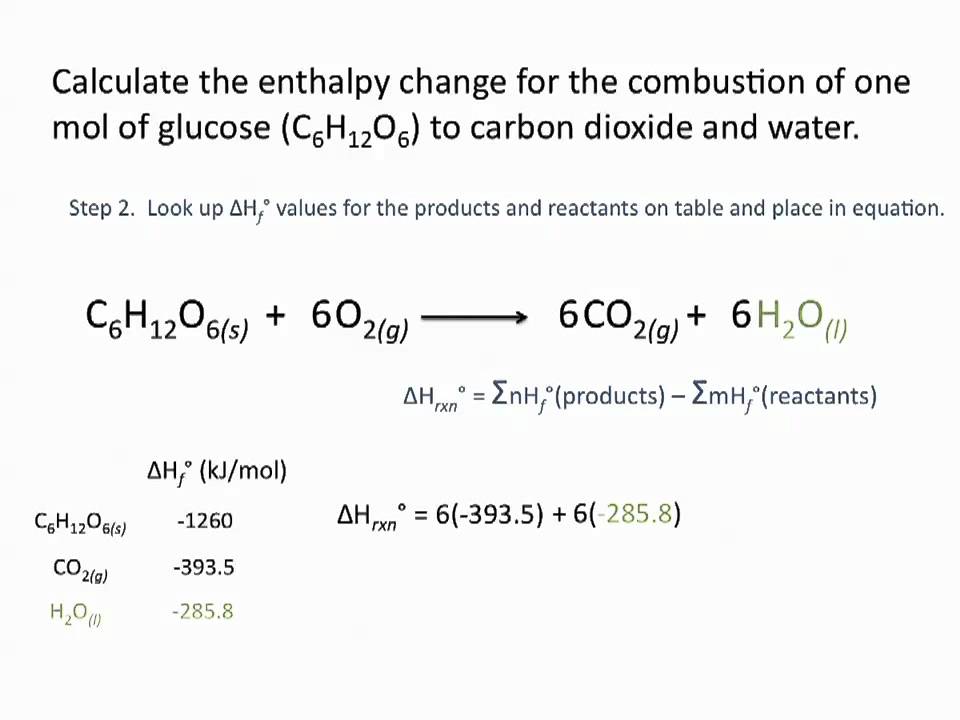
Calculate the standard enthalpy of formation of acetylene from the following data : C((g))+O(2(g))rarr CO(2(g)),DeltaH^(@)=-393kJ mol^(-1) H(2(g))+(1)/(2)O(2(g)) rarr H(2)O((l)),DeltaH^(@)=-285.8kJ mol^(-1) 2C(2)H(2(g))+5O(2(g))rarr 4CO(2(g))+2H(2)O((l ...
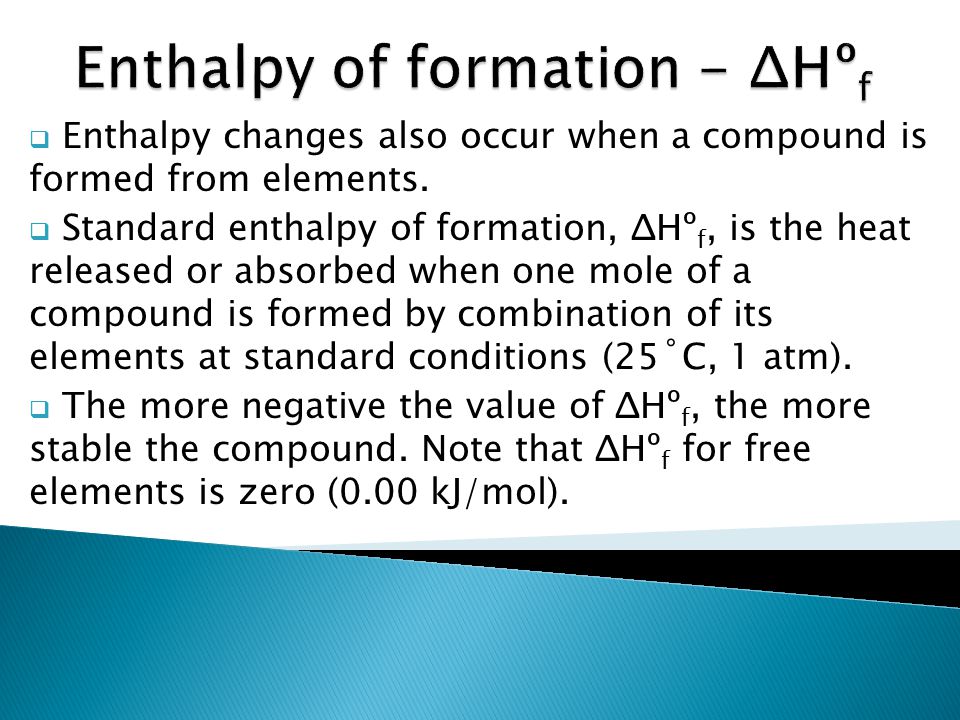
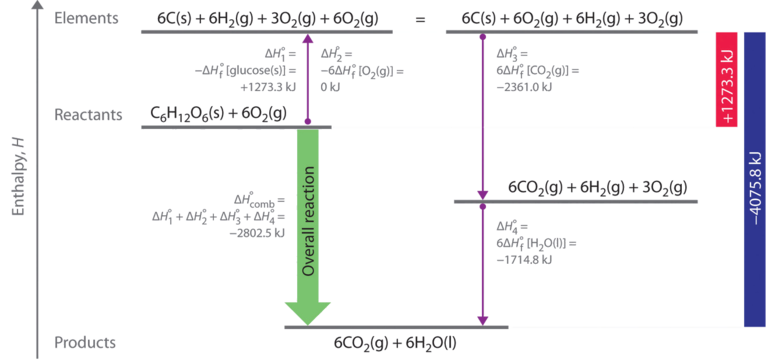
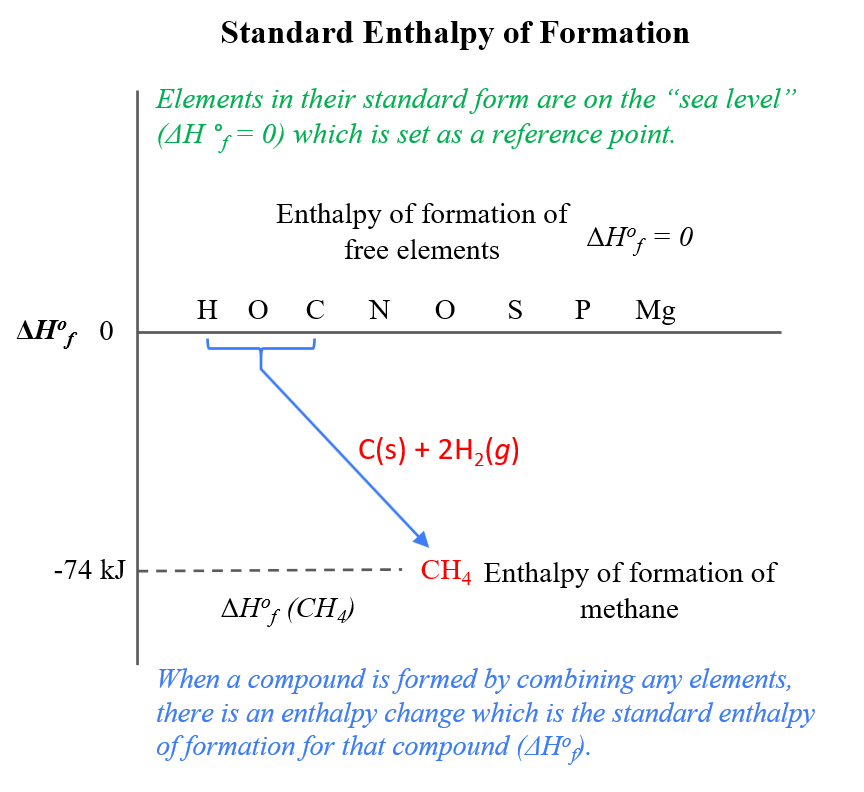
Enthalpy changes also occur when a compound is formed from elements. Standard enthalpy of formation, ΔHº f, is the heat released or absorbed when one. - ppt download

SOLVED: The combustion of tample of aluminium poduces 025 molof aluminium oxide and feleases 419.0 kJ of heat at standard conditions. Al () + % 0z (g) ++ B ALOs () Define